Question: Is it safe to ignite farts?
Short answer: Usually.
Long answer: Today’s question comes to us from a reader with a keen interest in pyroflatulence. This is the traditional practice of igniting farts, which has now been documented in a number of videos. The chemical principles behind this phenomenon have already been covered in an outstanding Wikipedia article, which we recommend highly to curious readers.
The question concerns the tradeoff between entertainment value and safety. There are many reported incidents of people injuring themselves while igniting farts, as well as at least one case of a fart causing an explosion during surgery.
Under normal circumstances, the issue comes down to positioning. To successfully light a fart, one has to place the flame close enough to the source to ignite the gas. But for the farter to escape injury, the flame has to be at a safe distance from clothes or anything else that might catch fire. As a public service, we have therefore attempted to evaluate the safety profile of pyroflatulence.
The ignitability of farts is probably due to their hydrogen sulfide content, which produces the characteristic fart smell and is also highly flammable. It has a lower flammability limit of 4.3%, which corresponds to the proportion of gas that must be present for ignition to take place.
In our experiments, a typical fart spreads slowly throughout the volume of our collection tube, eventually reaching an equilibrium:
For this fart, the equilibrium is at 3.9 ppm of hydrogen sulfide, which is equivalent to a concentration of 0.0004%. Of course, different farts have different equilibrium concentrations of hydrogen sulfide, but this one is close to the average in our sample. Importantly, its flammable gas concentration is far too weak to be ignited, in compliance with our laboratory safety protocols.
The volume of the collection tube is 2473 cm3, so in order to reach the lower limit of 4.3%, this same amount of fart gas would have to be compressed into a space 10,000 times smaller, or about 0.25 cm3. If we assume that farts are emitted as a cone of gas, the volume V of the cone is:
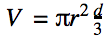
where d is the distance from the source. With a bit of algebra, we can estimate that the example fart would have to be ignited at a distance d of 0.62 cm. This is a small distance, but it is likely safe relative to the flame radius of most commercial lighters, which is around 0.5 cm.
For the farts in our database, we can therefore deduce the following relationship between fart smell and minimum ignitable distance:
The x-axis here is the equilibrium smelliness, as it is detected in our experiments. Unsurprisingly, smellier farts can be ignited at a larger distance from the source and are therefore safer to light. But with care, even a fart that would reach an equilibrium of 2.2 ppm in our collection tube can be ignited at the minimum distance of 0.5 cm (red dot in the graph above). This would include 66.9% of the farts in our database, so we conclude that about 2/3 of all farts can probably be ignited safely. But that means that 1/3 cannot, and so when lighting farts, it is important to be confident in one’s ability to produce a stink.
