Question: How much are fart sounds affected by atmospheric conditions?
Short answer: Not much.
Long answer: Robert Boyle was involved in a community of natural philosophers called an “Invisible College”; many of these scholars were later involved in the formation of the Royal Society in 1660. His best known scientific contribution is the formulation of Boyle’s law, which describes the relationship between the pressure and volume of a gas. As pressure goes up, volume goes down, leading to the simple relationship PV = k, where k is a constant. A subsequent generalization of this formulation is the Ideal Gas Law, which takes into account temperature as well: PV = nRT. Here n is the amount of substance, and R is the ideal gas constant. Rearranging this equation algebraically gives a general formulation for the volume of a gas: V = nRT/P.

Other things being equal, a fixed quantity of gas trapped in, say, someone’s intestines, will occupy a volume that increases with temperature and decreases with pressure. The latter relationship is known to hikers, as gassiness tends to become unpleasant during hikes at high altitude. The problem has been noted in medical investigations, which refer to it as High Altitude Flatus Explusion: “an increase in both the volume and the frequency of the passage of flatus, which spontaneously occurs while climbing to altitudes of 11,000 feet or greater”. A similar phenomenon has been noted during airplane flights, when cabin pressure drops.
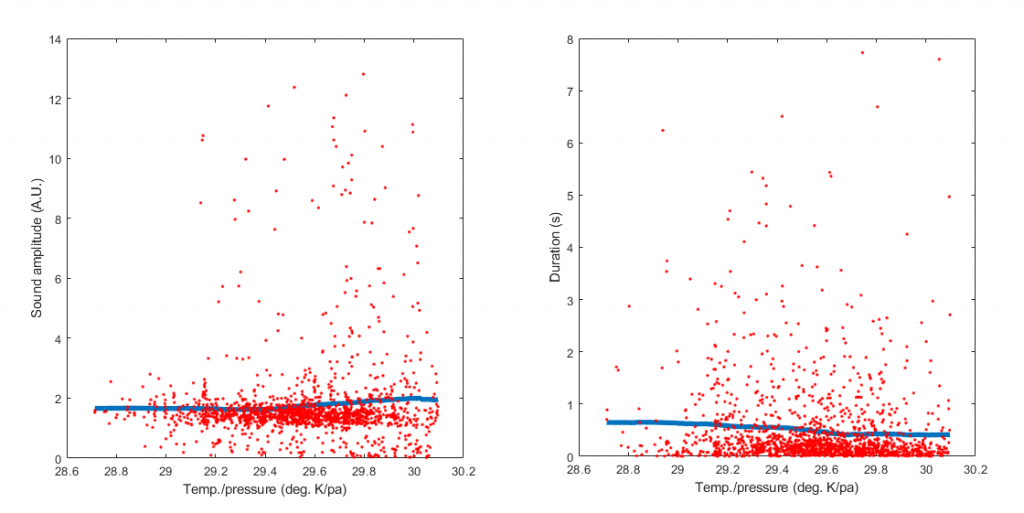
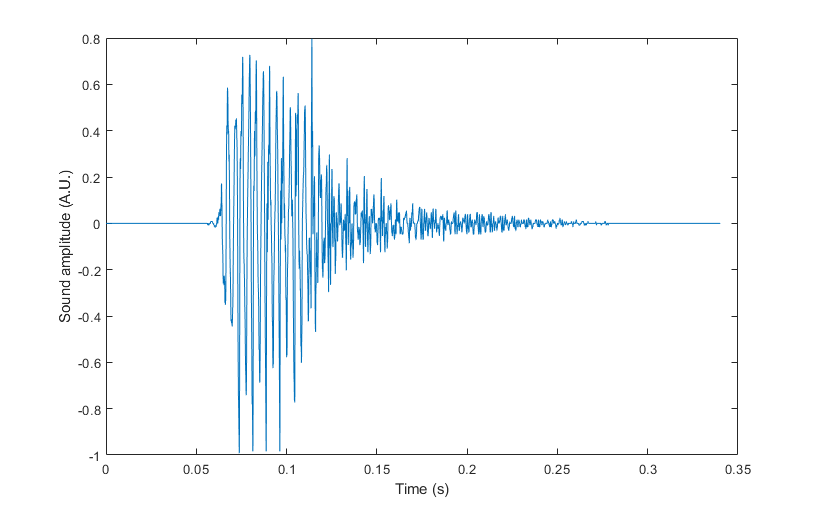
So, what effect does atmospheric pressure have on farts? A larger volume of gas might be expected to produce more noise as it is ejected through a fixed opening. We tested this hypothesis with a dataset comprised of 1619 farts. For each one, we had a sound recording (see figure below) that allowed us to extract the peak amplitude, and the duration, the latter being based on the envelope obtained from the Hilbert Transform of the waveform.
Below is a plot of T/P vs. loudness, which shows that the expected relationship does obtain – higher temperature and lower atmospheric pressure are associated with louder farts. Moreover, the T/P ratio is inversely related to fart duration (figure on the right). Both relationships are pretty weak, as we were limited to a fairly narrow range of atmospheric pressure and temperature. But these two findings do suggest that, other things being equal, the combination of high temperature and low pressure can lead to a rapid and noisy release of gas.