Question: Why can’t we see farts?
Short answer: It’s not cold enough.
Long answer: A previous post suggested that it should be possible to see farts, when the air temperature is below about 8 deg. Celsius. This was a theoretical calculation based on the observed temperature and humidity of farts, and the corresponding dew point. As is so often the case, the theory turned out to be wrong – we did our best on some moderately cold days, but no farts were detectable by eye. There is an obvious explanation for this, namely that the volume of water vapor released by a fart is too small to be seen.
But before we gave up on seeing a fart, we decided to check for flaws in the underlying theoretical framework. This required us to delve deeper into the field of psychrometrics, which is concerned with the mixing of gasses.
The amount of water released into the air by a fart can be expressed with the following equation:
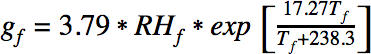
where RHf is the relative humidity of the fart and Tf is the temperature of the fart. The amount of water in the surrounding air is defined analogously:
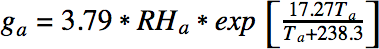
When the fart mixes with the ambient air the total amount of water (grams per kg of air) is given by g = (1 – m)gf + mga, where m is the mixing ratio. The mixing ratio defines the extent to which the fart has mixed with the surrounding air, and, critically, it was neglected in our earlier analysis. Clearly it is relevant – when the mixing ratio is too low, there is no chance of condensation, since the fart has not made contact with the outside air. When the mixing ratio is too high, the fart has diffused fully into the air and so it would not be visible. It follows that one should consider an intermediate mixing ratio of around 0.5.
So let’s consider a fart that is released with a typical relative humidity (RHf = 65%), at something close to body temperature (T = 33o). Incidentally, these values are similar to those measured for exhaled breath. Under these conditions the amount of water in the fart would be gf = 20.13 g/kg. Given the fact that a fart is typically about 100 ml of air, we can thus calculate that each fart contains about 0.0003 grams of water.
Substituting this into our equation for total amount of water, we then have:
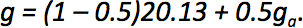
Since the total amount of water is conserved, we can find the values of Ta and RHa at which condensation would occur, using the following equation:
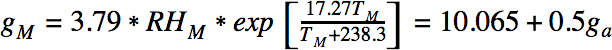
Condensation occurs at values of ga and TM for which RHm equals 100%.
The situation is actually a bit more complicated, because we have to consider the enthalpy of the fart-atmosphere system, as described here. We will spare the reader the details, but a handy calculator can be found here, for those who wish to work through the numbers.
In any case, using the values for a typical fart (RHf = 65% and T = 33o), we end up with the following predictions:
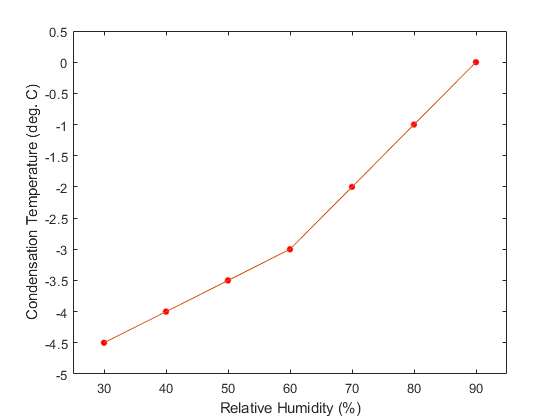
Thus, contrary to what you might have read, it is probably not possible to see farts when the ambient temperature is above about 0 deg. Celsius, and even then, the probability of success would depend strongly on the humidity of the air.
Being an experimental college, we have attempted to validate these calculations empirically. This required the construction of a new apparatus, the Event Horizon Flatoscope (EHF), which was capable of detecting the presence of farts, based only on the trail of water vapor they created. Below is one of the first successful measurements produced by the EHF, at an ambient temperature of – 7o C.

This breakthrough, like other less important ones, raises a host of questions for future research. But for now, we can say that it is indeed possible to visualize farts. Given our calculations and the available data on air humidity, the chance of success will be highest early in the morning on a very cold day.
